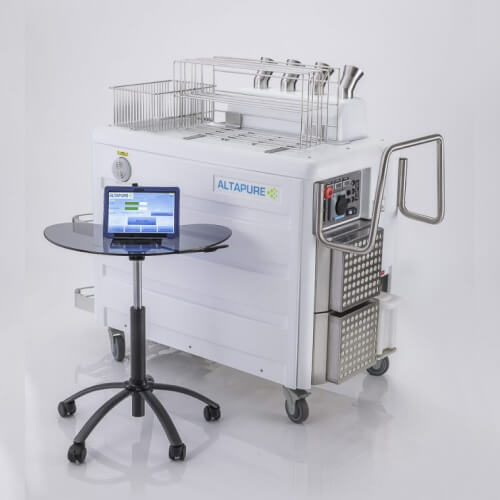
Complete solutions for pharmaceutical production
A complete range of solutions for pharmaceutical production: Environmental Monitoring Systems for Pharmaceutical Clean Rooms, Aerosol and Particle Monitoring, Aerosol Disinfection, Product Water Treatment

Pharmaceutical plants rely heavily on clean rooms to ensure the sterility and safety of their products. Clean rooms are controlled environments where contaminants such as dust, airborne microbes, aerosol particles, and chemical vapors are minimized. These rooms are essential for the production of drugs and vaccines, where even the slightest contamination can lead to compromised product quality and patient safety risks. The design of clean rooms involves the use of HEPA (High-Efficiency Particulate Air) filters to trap particles, maintaining positive air pressure to prevent the influx of contaminants, and stringent protocols for personnel entering the space, including the use of specialized garments.

Aerosol and particle monitoring in pharmaceutical plants is crucial for maintaining the integrity of clean rooms. This process involves the continuous measurement of particle concentration in the air to detect any deviation from acceptable levels. Particle counters and air samplers are commonly used tools, providing real-time data that help in identifying potential sources of contamination. Regular monitoring ensures that the air quality meets the strict regulatory standards set by agencies such as the FDA and EMA. Effective aerosol and particle monitoring helps in preempting contamination events, thereby safeguarding the manufacturing process.
Aerosol disinfection is a critical procedure in maintaining the sterility of clean rooms and other controlled environments within pharmaceutical plants. This method involves dispersing disinfectant aerosols throughout the clean room to eliminate microbial contamination on surfaces and in the air. Technologies such as vaporized hydrogen peroxide (VHP) and ultraviolet (UV) light systems are commonly used for this purpose. VHP is effective in reaching difficult areas and provides a uniform disinfection, while UV systems offer rapid disinfection capabilities. Regular aerosol disinfection is part of the comprehensive cleaning protocols that ensure a contaminant-free environment for pharmaceutical production.
Product water treatment is another vital component of pharmaceutical manufacturing. Water used in the production process must meet the highest purity standards as it can be a major source of contamination. Treatment systems typically include multiple stages such as filtration, reverse osmosis, deionization, and ultraviolet sterilization to remove impurities and microbial contaminants. The treated water is continuously monitored to ensure compliance with pharmacopeial standards, such as those outlined by the USP (United States Pharmacopeia). Proper water treatment is essential not only for the quality of the final product but also for the effectiveness and safety of the pharmaceuticals produced.
Products
Request a quote
You can trust the 10-year experience of our engineers in striving to provide the best solutions for your business!